The Environmental Impact of Refrigerant Gases

What is Global Warming?
Why is it warm in a greenhouse on a cool day? The answer is Ultra Violet (UN.). U.V. radiation penetrates the clouds with its medium frequency radiation waves. It can penetrate glass or perspex. Only when the U.V. hits an object, such as a flowerpot in a greenhouse does it turn from medium frequency U.V. to Long Wave Heat Radiation – that’s why it warms up.
In the atmosphere above the Earth there is a layer of naturally occurring gases which act as the glass in a greenhouse trapping heat around the Earth. This layer needs to be d correct thickness to enable life to survive. Gases produced within industry have added to the thickness of this layer of “Greenhouse Gases”. The most notable one being Carbon Dioxide (CO2).
Carbon Dioxide is a by-product of burning fossil fuels such as coal and oil in our power stations. There are approx. 7 billion tonnes of CO2 produced each year from creating electricity.
All refrigerant gasts (apart from Hydrocarbon’s) are extremely bad greenhouse gases. There are also other gases such as Methane that contribute to “Global Warming”.
The Environmental Impact Of
Refrigerant Gases
The U.V. from the Sun penetrates the Greenhouse Gases within the atmosphere and hit the Earth, The U.V. then changes energy to long wave radiation – heat. This heat cannot escape back through the Greenhouse Gases and we get Global Warming. The thicker the layer of Greenhouse Gases – the warmer the surface of the Earth becomes.
The average temperature around the Earth at present is + I5°C. If there were no Greenhouse Gases the average temperature would be -18°C. We do need some Global Warming to survive- but not too much.
Kyoto Protocol
In 1990 the countries that had signed up to the Montreal protocol held a meeting in Kyoto, Japan and agreed to reduce the emissions of gases with a Global Warming potential to a level of 5% less than the level in 1990. The UK has agreed to achieve a reduction of 12.5% below 1990 levels.
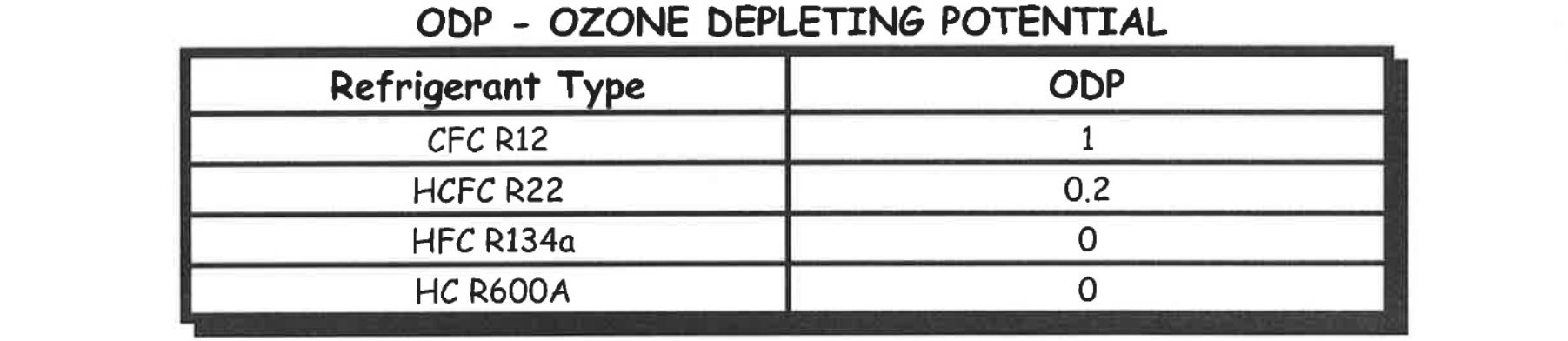
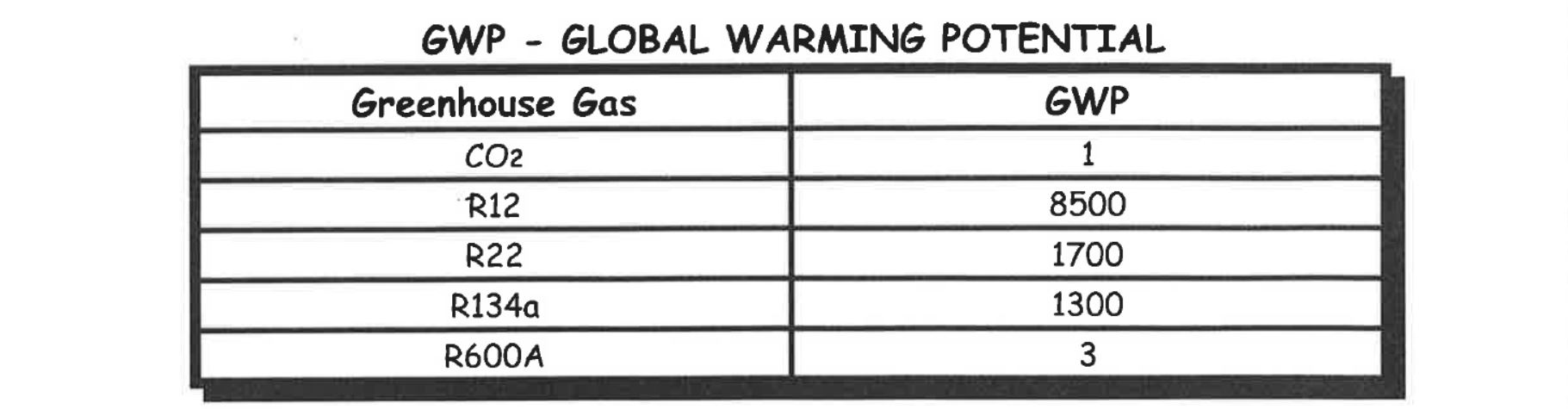
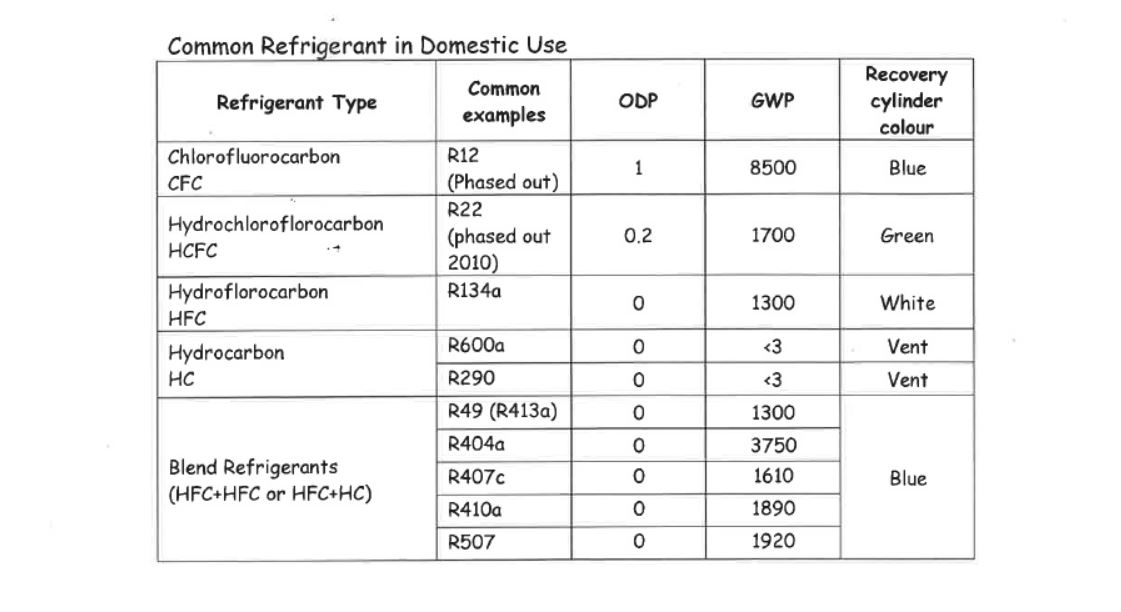
As you can see from the tables above all refrigerants have a global warming potential and/or an Ozone depleting potential, because of this no refrigerants except HC’s can be intentionally released to atmosphere.
INTENTIONAL RELEASE OF REFRIGERANT GASES TO ATMOSPHERE
CAN LEAD TO PROSECUTION AND LARGE FINES
The Environmental Impact Of
Refrigerant Gases
How the Ozone layer protects us from U.V.rays
The Ultra Violet Radiation from the Sun hits an Ozone molecule. In doing so it splits the Ozone molecule.
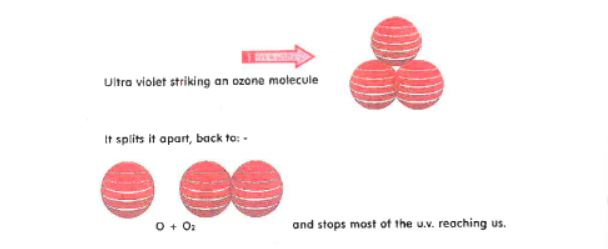
The cycle is then repeated. The energy from the Sun again joins the 0 and 02 to form Ozone 03.
So, this is a constant “split” and “rejoin” process. This is our protection against the dangerous Ultra Violet waves
Why is there a hole in the Ozone layer?
If refrigerant gas (CFC) leaks from a system it naturally drops to the ground because it is heavier than air. It gets up to the Stratosphere where the Ozone layer is by attaching itself to dirt,dust and debris and hitches a ride. It takes approx. 7 years for the CFC to reach the Stratosphere. Once the CFC molecule is up there, it is split apart by the U.V.
The Chlorine molecule breaks away from the Fluorine and Carbon molecules. This is now called “Free Chlorine”.
This Free Chlorine molecule attacks the Ozone and depletes it. It is estimated that one Free Chlorine atom can prevent the formation of 10,000 molecules of Ozone.
CFC refrigerants have a life span of approx. 100 years in the Stratosphere.

Ozone Depletion
The Ozone layer starts approx. 10km above the Earth and ends at approx. 50km above the Earth. Within this 40km wide band the scientists have determined there is approx. 95% of our Ozone (the other 5% is just outside this band).
The purpose of this Ozone layer is to protect us from harmful Ultra Violet radiation (U.V.). It acts rather like a shield, protecting humans, plant life and marine life.
In May of 1985, two British scientists discovered a hole in the Ozone layer at the South Pole. The fact is that the hole is now so large, it uncovers parts of Southern Chile. The people there need to take special precautions, as there is no “shield” of Ozone to protect them. They suffer from skin cancers and cataracts.
What is Ozone?
Ozone is a group of 3 Oxygen atoms – when all three join it becomes an Ozone molecule. The chemical symbol for Ozone is 03. Lets start by explaining the “joining” process.

A single Oxygen atom, unstable by itself will automatically join to another Oxygen atom to form 02 – Oxygen.
How is Ozone produced (O3)?
Another Oxygen atom will not automatically join with two atoms that have previously joined together, it needs some help.
The Sun emits a wide spectrum of magnetic radiation. It emits long wave radiation (heat), medium wave radiation (U.V.) and short wave radiation such as x-rays.
Between long wave and medium wave radiation there is a form of radiation energy, which is needed to create Ozone. This energy helps attach the third Oxygen atom to the two atoms that have previously joined together – to form Ozone.
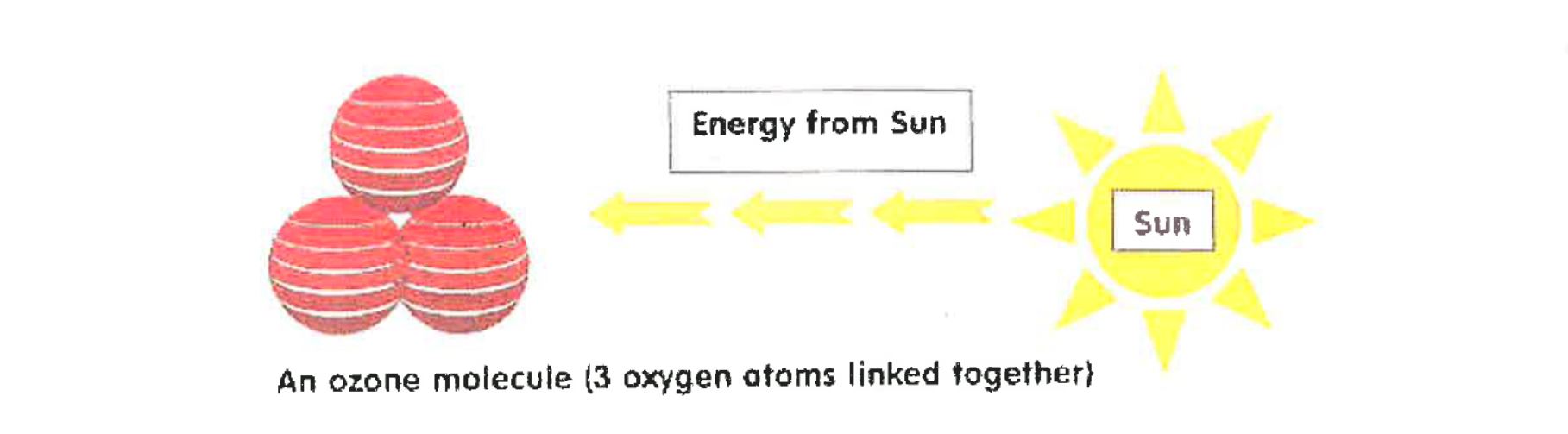
Ozone can only be produced when the Sun is out. That’s why at the end of the Antarctic winter, when there has been no Sun for up to 6 months, no Ozone has been produced and the hole appears. On the other hand, by the end of the Antarctic summer, when the Sun has been out for 24 hours every day for 6 months, the hole fills itself in. The hole has gradually increased in size over the years due to the increase in CFC (Chlorine, Flourine, Carbon) emissions.
The Institute of Refrigeration Codes of Practice
The institute of refrigeration have put in place guidelines on what refrigerant release is permissible under the Montreal and Kyoto agreements, this currently is covered under the Environmental Protection Act 1990, so there will be a specific Refrigerant Handling regulation requiring all people who use refrigerants to be trained and registered. Below are highlights taken from the institute of refrigeration codes of practice that relate to domestic refrigeration servicing. These guides cover all refrigerants except Hydrocarbons (R600a, R290)
Deliberate Venting
• Releasing refrigerant to atmosphere instead of full recovery
• Multiple evacuation of systems breaking each vacuum with refrigerant
n Using refrigerant to “blow out” blocked or restricted systems
• Adding refrigerant to a known leaking system to help locate a leak
• Release of refrigerant to cool cylinders to aid filling
Inadvertent Loss
n Small quantities from the normal connection and disconnection of gauge lines.
• Small loss from refrigerant oil after full recovery process has been completed